Quantum mechanics (nonfiction)
Quantum mechanics (QM; also known as quantum physics or quantum theory), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of energy levels of atoms and subatomic particles.
Classical physics (the physics existing before quantum mechanics) is a set of fundamental theories which describes nature at ordinary (macroscopic) scale. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale.
Quantum mechanics differs from classical physics in that: energy, momentum and other quantities of a system may be restricted to discrete values (quantization), objects have characteristics of both particles and waves (wave-particle duality), and there are limits to the precision with which quantities can be known (uncertainty principle).
Quantum mechanics gradually arose from the necessity of discrete energy values in Max Planck's solution in 1900 to the black-body radiation problem, and from the necessary correspondence between energy and frequency in Albert Einstein's 1905 paper which offered a quantum-based theory to explain the photoelectric effect.
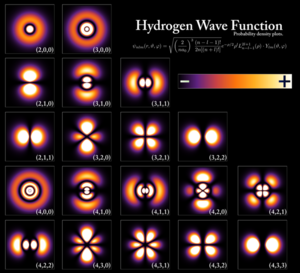
Early quantum theory was profoundly re-conceived in the mid-1920s by Erwin Schrödinger, Werner Heisenberg, Max Born and others. The modern theory is formulated in various mathematical formalisms. In one of them, a mathematical function, the wave function, provides information about the probability amplitude of position, momentum, and other physical properties of a particle.
Important applications of quantum theory include quantum chemistry, superconducting magnets, light-emitting diodes, and the laser, the transistor and semiconductors such as the microprocessor, medical and research imaging such as magnetic resonance imaging and electron microscopy. Explanations for many biological and physical phenomena are rooted in the nature of the chemical bond, most notably the macro-molecule DNA.
In the News
Fiction cross-reference
Nonfiction cross-reference
- Max Born (nonfiction)
- Albert Einstein (nonfiction)
- Ghost (nonfiction) - In the terminology of quantum field theory, a ghost, ghost field, or gauge ghost is an unphysical state in a gauge theory. Ghosts are necessary to keep gauge invariance in theories where the local fields exceed a number of physical degrees of freedom.
- Werner Heisenberg (nonfiction)
- Hidden-measurements interpretation (nonfiction)
- Quantum eraser (nonfiction)
- Physics (nonfiction)
- Max Planck (nonfiction)
- Erwin Schrödinger (nonfiction)
- Uncertainty principle (nonfiction)
External links:
- Quantum mechanics @ Wikipedia